A new polymer gel can deliver insulin through intact skin in animal tests. It could someday offer a path toward needle-free diabetes treatment, some say.



He’s not alone. xAI’s head of compute has reportedly bet his counterpart at Anthropic that 1% of global compute will be in orbit by 2028. Google (which has a significant ownership stake in SpaceX) has announced a space AI effort called Project Suncatcher, which will launch prototype vehicles in 2027. Starcloud, a startup that has raised $34 million backed by Google and Andreessen Horowitz, filed its own plans for an 80,000 satellite constellation last week. Even Jeff Bezos has said this is the future.
But behind the hype, what will it actually take to get data centers into space?
In a first analysis, today’s terrestrial data centers remain cheaper than those in orbit. Andrew McCalip, a space engineer, has built a helpful calculator comparing the two models. His baseline results show that a 1 GW orbital data center might cost $42.4 billion — almost 3x its ground-bound equivalent, thanks to the up-front costs of building the satellites and launching them to orbit.

WASHINGTON (AP) — Scientists are testing an entirely new way to fight heart disease: a gene-editing treatment that might offer a one-time fix for high cholesterol.
It’s very early stage research, tried in only a few dozen people so far. But gene-editing approaches being developed by two companies show hints that switching off certain genes could dramatically lower artery-clogging cholesterol, raising hopes of one day being able to prevent heart attacks without having to take pills.
“People want a fix, not a bandage,” said Dr. Luke Laffin, a preventive cardiologist at the Cleveland Clinic. After co-authoring a promising study published in the New England Journal of Medicine, he said he was flooded with queries about how to participate in the next clinical trial.

A multi-institutional team of researchers led by Virginia Tech’s Fralin Biomedical Research Institute at VTC has for the first time identified specific patterns of brain chemical activity that predict how quickly individual honey bees learn new associations, offering important insights into the biological basis of learning and decision-making. The study, published in Science Advances, found that the balance between the neurotransmitters octopamine and tyramine can predict whether a bee will learn quickly, slowly, or not at all, as they associate an odor with a reward.
Because the same ancient brain chemicals that guide learning in bees also shape attention and learning in people, the findings may help scientists better understand why individual humans learn at different speeds—and how those processes may go awry in a variety of brain disorders.
Specific patterns of brain chemical activity appear before learning begins and again when a learned behavior first emerges, signaling how quickly an individual bee will learn. The research can help explain how chemicals in the brain drive attention and reinforce learning, with implications for fundamental biology, medicine, and agriculture.
NASA has advanced plans for a 500-kilowatt (kW) lunar nuclear reactor to power astronauts, Moon bases and deep space missions by 2030.


Found in everything from protein bars to energy drinks, erythritol has long been considered a safe alternative to sugar.
But research suggests this widely used sweetener may be quietly undermining one of the body’s most crucial protective barriers – with potentially serious consequences for heart health and stroke risk.
A study from the University of Colorado suggests erythritol may damage cells in the blood-brain barrier, the brain’s security system that keeps out harmful substances while letting in nutrients.
Elon Musk Announces MAJOR Company Changes as XAI/SpaceX ## Elon Musk is announcing significant changes and advancements across his companies, primarily focused on developing and integrating artificial intelligence (AI) to drive innovation, productivity, and growth ## ## Questions to inspire discussion.
Product Development & Market Position.
🚀 Q: How fast did xAI achieve market leadership compared to competitors?
A: xAI reached number one in voice, image, video generation, and forecasting with the Grok 4.20 model in just 2.5 years, outpacing competitors who are 5–20 years old with larger teams and more resources.
📱 Q: What scale did xAI’s everything app reach in one year?
A: In one year, xAI went from nothing to 2M Teslas using Grok, deployed a Grok voice agent API, and built an everything app handling legal questions, slide decks, and puzzles.

ENeuro: Ringelberg et al. identify a key role for excitatory neuron loss of UBE3A in motor, innate, and sleep behavioral phenotypes of Angelman syndrome model mice.
▶️
AS is a neurodevelopmental disorder with no disease-modifying treatment. However, clinical trials are currently underway using antisense oligonucleotides to unsilence the dormant paternal UBE3A allele, thereby normalizing UBE3A levels (Ionis: NCT05127226; Ultragenyx: NCT04259281). While this approach holds exciting promise and shows efficacy in mouse models (Meng et al., 2015; Milazzo et al., 2021), there is currently scant information regarding the key cell types or brain regions that require UBE3A reinstatement to mitigate core symptoms of AS. This holds particular importance, as effective biodistribution is a key concern in genetic therapies for CNS disorders (Roberts et al., 2020; Jafar-Nejad et al., 2021; Ling et al., 2023), and suboptimal targeting of necessary cell classes could hamper success. Moreover, mouse models of AS require early postnatal Ube3a reinstatement to achieve optimal phenotypic recovery (Silva-Santos et al., 2015; Sonzogni et al., 2020); early intervention could be difficult to achieve in the patient population without a corresponding early diagnosis, meaning many AS individuals are likely beyond the critical window to maximally benefit from UBE3A reinstatement-based therapies. Therefore, additional work is needed to better understand how loss of UBE3A leads to symptoms, as these insights will aid both in understanding the cell types that must be targeted for optimal genetic interventions and in developing alternative therapeutic options.
Our laboratory’s previous work identified an outsized role of GABAergic loss of UBE3A in hyperexcitability phenotypes. GABAergic loss of UBE3A drives increased delta power on cortical EEG (Judson et al., 2016), a phenotype that correlates with the severity of a range of symptoms in AS individuals (Hipp et al., 2021; Ostrowski et al., 2021). Further, mice with Ube3a deleted from GABAergic neurons show decreased threshold to chemically and acoustically driven seizures, and they also exhibit spontaneous behavioral seizures, a phenotype not observed in AS model mice on a C57BL/6J background (Judson et al., 2016; Gu et al., 2019). These data forewarn that UBE3A reinstatement in a manner biased to glutamatergic neurons could potentially worsen epilepsy-related symptoms and highlight the importance of studying the neuronal populations regulating other behaviors.
Based on the exaggerated role of GABAergic neurons in AS seizure phenotypes, we predicted that GABAergic deletion of Ube3a would underlie a broad range of behavioral phenotypes in AS mice. In the present study, we instead found a larger role of Ube3a deletion from glutamatergic neurons in motor coordination, measured by rotarod and open field behavior, and innate species-specific behaviors such as marble burying. Furthermore, glutamatergic loss of UBE3A appears to mediate alterations in sleep patterning and induces some sleep fragmentation, while UBE3A loss from GABAergic neurons only caused fragmented sleep. Interestingly, glutamatergic reinstatement of Ube3a also rescued the decreased REM sleep observed in AS mice, as estimated by the PiezoSleep system. While this study identified some roles of GABAergic neurons in nest building behavior and sleep fragmentation, our data largely suggest a divergence of the neural circuitry underlying the motor, innate behavior, and sleep phenotypes of AS mice from the circuitry responsible for seizure susceptibility and cortical EEG patterns.
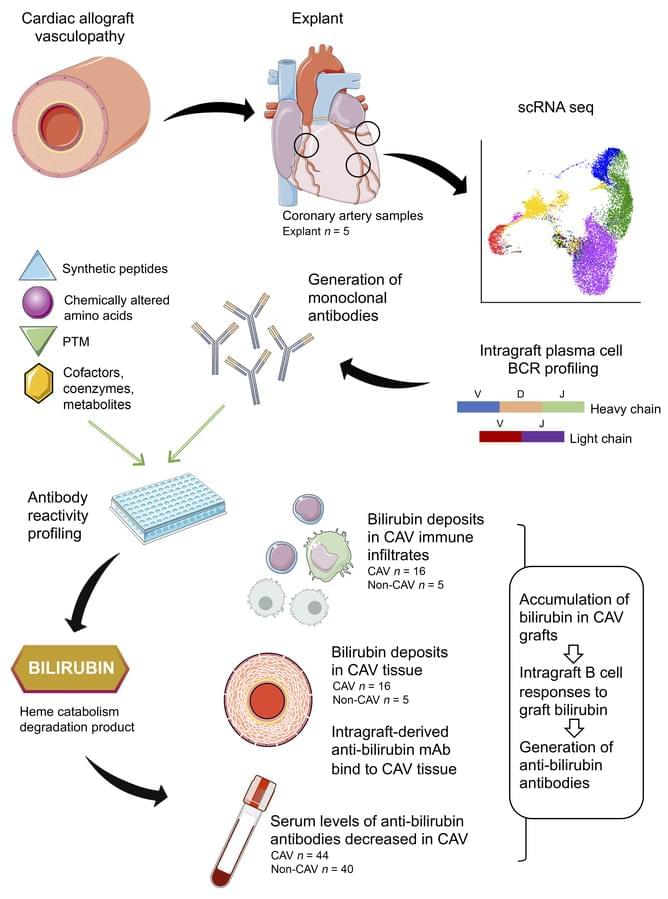
Emmanuel Zorn & team discover plasma cells in human cardiac allografts with vasculopathy target bilirubin, revealing local heme catabolism:
The image shows immunofluorescence staining for bilirubin (green) and a-smooth muscle cell actin (red) of cardiac tissue with CAV.
1Columbia University Irving Medical Center, New York, New York, USA.
2Institute of Anatomy and Cell Biology, Faculty of Medicine, Martin-Luther-University Halle-Wittenberg, Halle, Germany.
3Kidney Transplant Unit, Nephrology Department, Vall d’Hebron University Hospital, Barcelona, Spain.